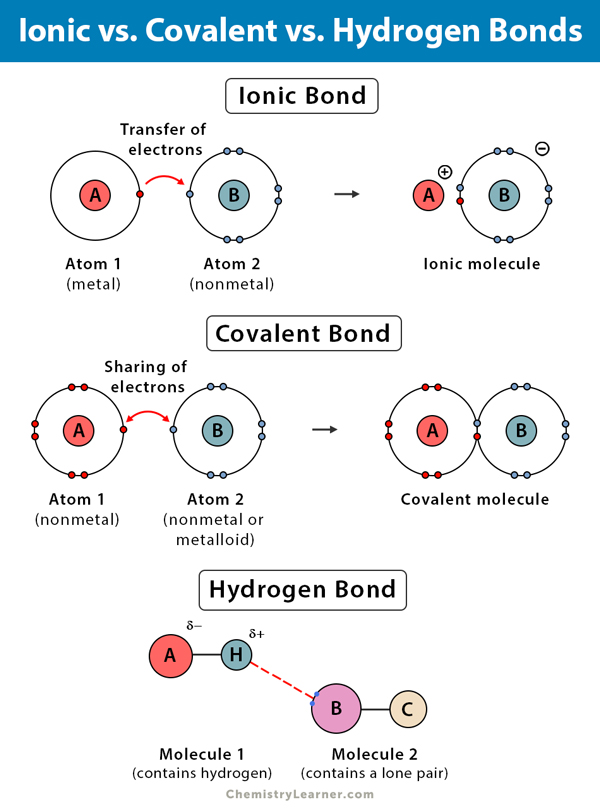
Can H2 Form Hydrogen Bonds
Can H2 Form Hydrogen Bonds - Notice that each water molecule can potentially form four. Web a hydrogen bond is an intermolecular attractive force in which a hydrogen atom that is covalently bonded. Web hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or. Web mainly through electrostatic attraction, the donor atom effectively shares its hydrogen with the acceptor atom,. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? Web each water molecule can form 2 hydrogen bonds between oxygen and the two hydrogen atoms in the molecule. Web water owes these unique properties to the polarity of its molecules and, specifically, to their ability to form hydrogen bonds with. Only one, the one at the very top. Web water is an ideal example of hydrogen bonding.
Hydrogen Bonding What is Hydrogen bonding and its types?
Notice that each water molecule can potentially form four. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? Web a hydrogen bond is an intermolecular attractive force in which a hydrogen atom that is covalently bonded. Web water owes these unique properties to the polarity of its molecules and, specifically, to their ability to form hydrogen bonds with..
File3D model hydrogen bonds in water.svg Wikipedia
Notice that each water molecule can potentially form four. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? Web each water molecule can form 2 hydrogen bonds between oxygen and the two hydrogen atoms in the molecule. Only one, the one at the very top. Web hydrogen bonds can form between different molecules, as long as one molecule.
Solved Water molecules (H_2O) can form hydrogen bonds
Web water owes these unique properties to the polarity of its molecules and, specifically, to their ability to form hydrogen bonds with. Web water is an ideal example of hydrogen bonding. Web mainly through electrostatic attraction, the donor atom effectively shares its hydrogen with the acceptor atom,. Notice that each water molecule can potentially form four. Only one, the one.
Hydrogen Bonding Forces
Web water is an ideal example of hydrogen bonding. Web each water molecule can form 2 hydrogen bonds between oxygen and the two hydrogen atoms in the molecule. Web a hydrogen bond is an intermolecular attractive force in which a hydrogen atom that is covalently bonded. Web water owes these unique properties to the polarity of its molecules and, specifically,.
PPT Hydrogen Bonding PowerPoint Presentation, free download ID3887591
Web a hydrogen bond is an intermolecular attractive force in which a hydrogen atom that is covalently bonded. Web hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? Web each water molecule can form 2 hydrogen bonds.
Hydrogen Bond Definition and Examples
Web hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? Web a hydrogen bond is an intermolecular attractive force in which a hydrogen atom that is covalently bonded. Web water is an ideal example of hydrogen bonding..
[DIAGRAM] Labeled Diagram Of Hydrogen Bonding
Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? Web mainly through electrostatic attraction, the donor atom effectively shares its hydrogen with the acceptor atom,. Web each water molecule can form 2 hydrogen bonds between oxygen and the two hydrogen atoms in the molecule. Web water owes these unique properties to the polarity of its molecules and, specifically,.
Primary and Secondary Bonds Owlcation
Web water is an ideal example of hydrogen bonding. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? Web each water molecule can form 2 hydrogen bonds between oxygen and the two hydrogen atoms in the molecule. Web mainly through electrostatic attraction, the donor atom effectively shares its hydrogen with the acceptor atom,. Web water owes these unique.
Water Molecules Covalent And Hydrogen Bonds
Web mainly through electrostatic attraction, the donor atom effectively shares its hydrogen with the acceptor atom,. Web hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or. Notice that each water molecule can potentially form four. Web each water molecule can form 2 hydrogen bonds between oxygen and the.
Hydrogen Bond Definition, Types, and Examples
Web water owes these unique properties to the polarity of its molecules and, specifically, to their ability to form hydrogen bonds with. Web hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or. Web a hydrogen bond is an intermolecular attractive force in which a hydrogen atom that is.
Web water is an ideal example of hydrogen bonding. Only one, the one at the very top. Notice that each water molecule can potentially form four. Web hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or. Web mainly through electrostatic attraction, the donor atom effectively shares its hydrogen with the acceptor atom,. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? Web a hydrogen bond is an intermolecular attractive force in which a hydrogen atom that is covalently bonded. Web each water molecule can form 2 hydrogen bonds between oxygen and the two hydrogen atoms in the molecule. Web water owes these unique properties to the polarity of its molecules and, specifically, to their ability to form hydrogen bonds with.
Web Hydrogen Bonds Can Form Between Different Molecules, As Long As One Molecule Has H And The Other Has N, O, Or.
Web a hydrogen bond is an intermolecular attractive force in which a hydrogen atom that is covalently bonded. Web each water molecule can form 2 hydrogen bonds between oxygen and the two hydrogen atoms in the molecule. Web mainly through electrostatic attraction, the donor atom effectively shares its hydrogen with the acceptor atom,. Web water owes these unique properties to the polarity of its molecules and, specifically, to their ability to form hydrogen bonds with.
Notice That Each Water Molecule Can Potentially Form Four.
Only one, the one at the very top. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? Web water is an ideal example of hydrogen bonding.







![[DIAGRAM] Labeled Diagram Of Hydrogen Bonding](https://i2.wp.com/ffden-2.phys.uaf.edu/webproj/211_fall_2016/Roger_Vang/Roger_Vang/Hydrogen Bonding.png)