Can Nonpolar Molecules Form Hydrogen Bonds
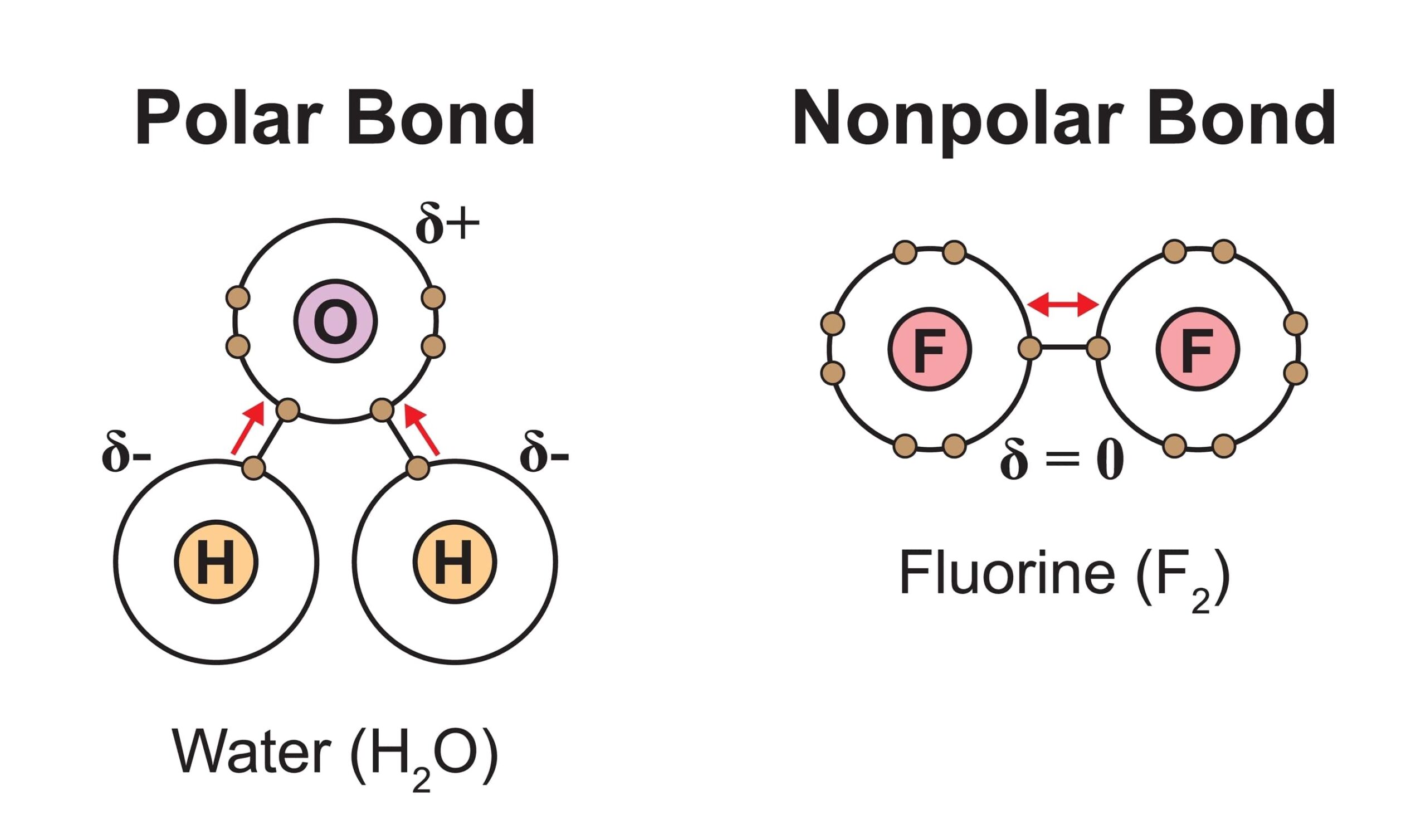
Can Nonpolar Molecules Form Hydrogen Bonds - Web this, without taking hydrogen bonds into account, is due to greater dispersion forces (see interactions. Web hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or f. In this video, we're going. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? Only one, the one at the very top. Web in the video on electronegativity, we learned how to determine whether a covalent bond is polar or nonpolar. Web a covalent bond that has an equal sharing of electrons (part (a) of figure \(\pageindex{1}\)) is called a nonpolar covalent bond.
Solubility of Organic Compounds Chemistry Steps
Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? Web this, without taking hydrogen bonds into account, is due to greater dispersion forces (see interactions. Web a covalent bond that has an equal sharing of electrons (part (a) of figure \(\pageindex{1}\)) is called a nonpolar covalent bond. Only one, the one at the very top. In this video,.
Hydrogen Bond
Web hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or f. In this video, we're going. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? Only one, the one at the very top. Web in the video on electronegativity, we learned how to determine whether a.
Understanding Types of Chemical Bonds NurseHub
Web in the video on electronegativity, we learned how to determine whether a covalent bond is polar or nonpolar. Web a covalent bond that has an equal sharing of electrons (part (a) of figure \(\pageindex{1}\)) is called a nonpolar covalent bond. Only one, the one at the very top. Web hydrogen bonds can form between different molecules, as long as.
Difference Between Intermolecular and Intramolecular Hydrogen Bonding Definition, Features and
Web in the video on electronegativity, we learned how to determine whether a covalent bond is polar or nonpolar. Only one, the one at the very top. In this video, we're going. Web a covalent bond that has an equal sharing of electrons (part (a) of figure \(\pageindex{1}\)) is called a nonpolar covalent bond. Web this, without taking hydrogen bonds.
CHEMICAL BONDS pediagenosis
Web in the video on electronegativity, we learned how to determine whether a covalent bond is polar or nonpolar. In this video, we're going. Only one, the one at the very top. Web this, without taking hydrogen bonds into account, is due to greater dispersion forces (see interactions. Web hydrogen bonds can form between different molecules, as long as one.
Polar and Nonpolar Molecules
Web a covalent bond that has an equal sharing of electrons (part (a) of figure \(\pageindex{1}\)) is called a nonpolar covalent bond. Only one, the one at the very top. Web in the video on electronegativity, we learned how to determine whether a covalent bond is polar or nonpolar. In this video, we're going. Web hydrogen bonds can form between.
SOLVEDCan nonpolar molecules such as CH4 participate in hydrogen bonds? Why or why not?
Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? In this video, we're going. Web in the video on electronegativity, we learned how to determine whether a covalent bond is polar or nonpolar. Web hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or f. Web this,.
Solved Water molecules (H_2O) can form hydrogen bonds
In this video, we're going. Only one, the one at the very top. Web hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or f. Web this, without taking hydrogen bonds into account, is due to greater dispersion forces (see interactions. Web how many hydrogens in figure \(\pageindex{1}\) can.
PPT Hydrogen Bonding PowerPoint Presentation, free download ID3887591
In this video, we're going. Web this, without taking hydrogen bonds into account, is due to greater dispersion forces (see interactions. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? Web hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or f. Web in the video on.
PPT Covalent Compounds PowerPoint Presentation, free download ID2169970
Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? Web in the video on electronegativity, we learned how to determine whether a covalent bond is polar or nonpolar. Only one, the one at the very top. Web hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or.
Web this, without taking hydrogen bonds into account, is due to greater dispersion forces (see interactions. Web in the video on electronegativity, we learned how to determine whether a covalent bond is polar or nonpolar. Web hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or f. Only one, the one at the very top. Web how many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? In this video, we're going. Web a covalent bond that has an equal sharing of electrons (part (a) of figure \(\pageindex{1}\)) is called a nonpolar covalent bond.
Web How Many Hydrogens In Figure \(\Pageindex{1}\) Can Form Hydrogen Bonds?
Only one, the one at the very top. Web hydrogen bonds can form between different molecules, as long as one molecule has h and the other has n, o, or f. Web this, without taking hydrogen bonds into account, is due to greater dispersion forces (see interactions. Web a covalent bond that has an equal sharing of electrons (part (a) of figure \(\pageindex{1}\)) is called a nonpolar covalent bond.
Web In The Video On Electronegativity, We Learned How To Determine Whether A Covalent Bond Is Polar Or Nonpolar.
In this video, we're going.