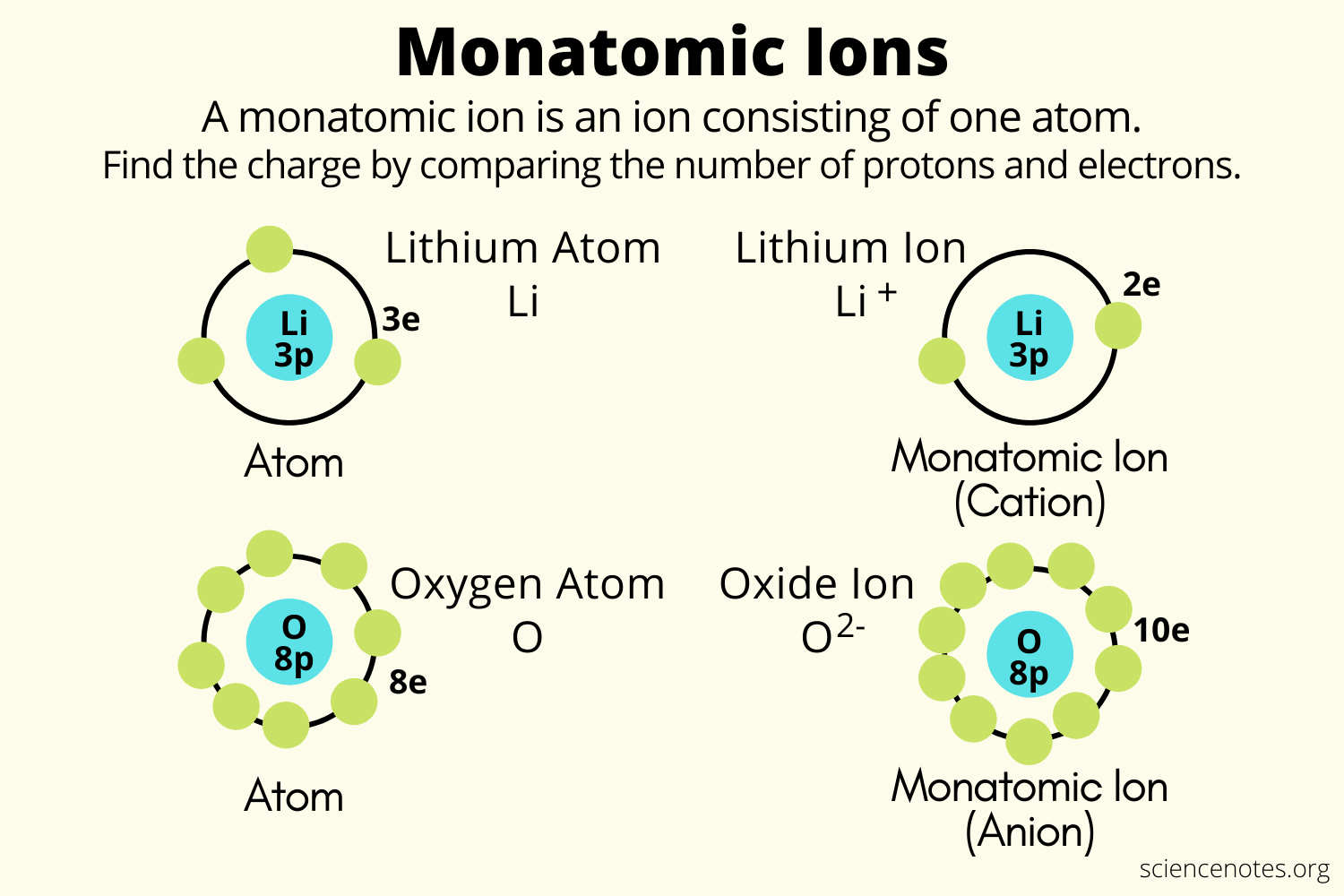
Oxygen Forms Monatomic Ions With A Charge Of
Oxygen Forms Monatomic Ions With A Charge Of - Web compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. After learning a few more details about the. Ions formed from a single atom. Web atoms of group 17 gain one electron and form anions with a 1− charge; Determine the number of valence. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. 100% (1 rating) share share. Atoms form this monatomic ions so they have the most stable electron configuration. Web name monoatomic ions using the defined nomenclature rules. Web the ions that we have discussed so far are called monatomic ions, that is, they are ions formed from only one.
Monatomic Ion Definition and Examples
Web atoms naturally form monatomic ions during chemical reactions, when ionic compounds melt, and when electrolytes dissociate in water. 100% (1 rating) share share. Web atoms of group 17 gain one electron and form anions with a 1− charge; Atoms form this monatomic ions so they have the most stable electron configuration. Here’s how to approach this question.
3.2 Ions The Basics of General, Organic, and Biological Chemistry
After learning a few more details about the. Ions formed from a single atom. 100% (1 rating) share share. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. Web atoms naturally form monatomic ions during chemical reactions, when ionic compounds melt, and when electrolytes dissociate in water.
Monatomic Ions
100% (1 rating) share share. Web in this section we will focus on monatomic ions: Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. Later in this chapter we will look at. Web the ions that we have discussed so far are called monatomic ions, that is, they are ions formed from.
Solved A monatomic ion with a charge of +1 has an electronic
Web in this section we will focus on monatomic ions: Later in this chapter we will look at. Web atoms of group 17 gain one electron and form anions with a 1− charge; After learning a few more details about the. Ions formed from a single atom.
Predicting Charge of Monatomic Ions
After learning a few more details about the. Web atoms naturally form monatomic ions during chemical reactions, when ionic compounds melt, and when electrolytes dissociate in water. Web atoms of group 17 gain one electron and form anions with a 1− charge; Determine the number of valence. Web name monoatomic ions using the defined nomenclature rules.
PPT Monatomic Ions PowerPoint Presentation, free download ID6687141
Determine the number of valence. 100% (1 rating) share share. Web name monoatomic ions using the defined nomenclature rules. Web compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. Here’s how to approach this question.
Common Charges of Monatomic Ions YouTube
100% (1 rating) share share. Here’s how to approach this question. Web in this section we will focus on monatomic ions: Web the ions that we have discussed so far are called monatomic ions, that is, they are ions formed from only one. Web name monoatomic ions using the defined nomenclature rules.
PPT Nomenclature PowerPoint Presentation, free download ID5771779
100% (1 rating) share share. Web atoms of group 17 gain one electron and form anions with a 1− charge; Web atoms naturally form monatomic ions during chemical reactions, when ionic compounds melt, and when electrolytes dissociate in water. Web name monoatomic ions using the defined nomenclature rules. Here’s how to approach this question.
Naming Monatomic and Polyatomic Ions Chemistry Steps
Determine the number of valence. Ions formed from a single atom. 100% (1 rating) share share. Web in this section we will focus on monatomic ions: Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on.
A monatomic ion with a charge of 1 has an electr…
Atoms form this monatomic ions so they have the most stable electron configuration. Determine the number of valence. After learning a few more details about the. Ions formed from a single atom. Web in this section we will focus on monatomic ions:
Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. Atoms form this monatomic ions so they have the most stable electron configuration. Web atoms of group 17 gain one electron and form anions with a 1− charge; Ions formed from a single atom. Web the ions that we have discussed so far are called monatomic ions, that is, they are ions formed from only one. Later in this chapter we will look at. Here’s how to approach this question. Web compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. 100% (1 rating) share share. Web name monoatomic ions using the defined nomenclature rules. Web in this section we will focus on monatomic ions: After learning a few more details about the. Web atoms naturally form monatomic ions during chemical reactions, when ionic compounds melt, and when electrolytes dissociate in water. Determine the number of valence.
Ions Formed From A Single Atom.
Web in this section we will focus on monatomic ions: Web atoms naturally form monatomic ions during chemical reactions, when ionic compounds melt, and when electrolytes dissociate in water. After learning a few more details about the. Web the ions that we have discussed so far are called monatomic ions, that is, they are ions formed from only one.
Atoms Of Group 16 Gain Two Electrons And Form Ions With A 2− Charge, And So On.
Here’s how to approach this question. 100% (1 rating) share share. Web compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. Determine the number of valence.
Web Atoms Of Group 17 Gain One Electron And Form Anions With A 1− Charge;
Web name monoatomic ions using the defined nomenclature rules. Atoms form this monatomic ions so they have the most stable electron configuration. Later in this chapter we will look at.