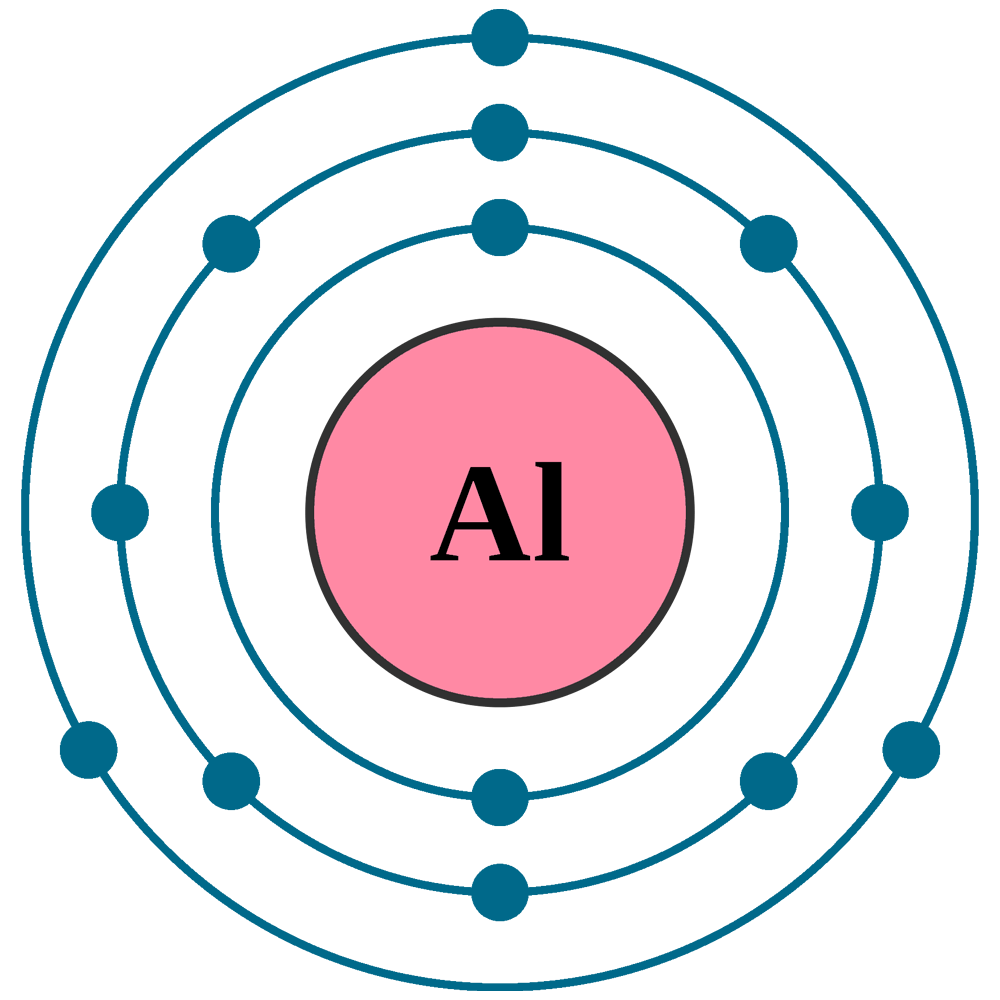
What Is The Charge On The Ion Formed By Aluminum
What Is The Charge On The Ion Formed By Aluminum - Web the ionic charge of aluminum (al) is 3+. Web aluminum and carbon react to form an ionic compound. Web the charge of an aluminum ion is typically 3+. Predict which forms an anion, which forms a cation, and the. Web using a simple, general trend for the ionic charge for elements on the periodic table, in this video we find the ionic charge for. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. This is because the element's atomic number is 13, reflecting the fact that it has. Web the aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. Web aluminum and carbon react to form an ionic compound. But the question is how can you find the ionic charge on aluminum (al)?.
What is the Charge on the Ion Formed by Aluminum? Aluminum Profile Blog
Web using a simple, general trend for the ionic charge for elements on the periodic table, in this video we find the ionic charge for. Predict which forms an anion, which forms a cation, and the. Web the charge of an aluminum ion is typically 3+. But the question is how can you find the ionic charge on aluminum (al)?..
Electron Configuration for Aluminum (Al, Al3+ ion)
For example, iron( ii ) has a. Predict which forms an anion, which forms a cation, and the charges of each. Web the aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. This is because the element's atomic number is 13, reflecting the fact that it has. Web roman numeral notation indicates.
What is the Charge on the Ion Formed by Aluminum? Aluminum Profile Blog
This is because the element's atomic number is 13, reflecting the fact that it has. Web roman numeral notation indicates charge of ion when element commonly forms more than one ion. Web the ionic charge of aluminum (al) is 3+. Web aluminum and carbon react to form an ionic compound. Predict which forms an anion, which forms a cation, and.
Aluminium Al (Element 13) of Periodic Table Elements FlashCards
Web aluminum and carbon react to form an ionic compound. Web the ionic charge of aluminum (al) is 3+. Predict which forms an anion, which forms a cation, and the. Web the aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. Web aluminum and carbon react to form an ionic compound.
How to Find the Ionic Charge for Aluminum (Al) YouTube
Predict which forms an anion, which forms a cation, and the. This is because the element's atomic number is 13, reflecting the fact that it has. Predict which forms an anion, which forms a cation, and the charges of each. For example, iron( ii ) has a. Web roman numeral notation indicates charge of ion when element commonly forms more.
5.2.1 Formation of Ion Revision.my
Predict which forms an anion, which forms a cation, and the charges of each. Web aluminum and carbon react to form an ionic compound. For example, iron( ii ) has a. Web the charge of an aluminum ion is typically 3+. Web roman numeral notation indicates charge of ion when element commonly forms more than one ion.
PPT 1 Name the ions formed by these elements and classify them as anions or cations
Web the ionic charge of aluminum (al) is 3+. Web the charge of an aluminum ion is typically 3+. But the question is how can you find the ionic charge on aluminum (al)?. Web using a simple, general trend for the ionic charge for elements on the periodic table, in this video we find the ionic charge for. Web roman.
What is the Charge on the Ion Formed by Aluminum? Aluminum Profile Blog
Web the ionic charge of aluminum (al) is 3+. Predict which forms an anion, which forms a cation, and the. Web the charge of an aluminum ion is typically 3+. Predict which forms an anion, which forms a cation, and the charges of each. This is because the element's atomic number is 13, reflecting the fact that it has.
PPT 1 Name the ions formed by these elements and classify them as anions or cations
Web the aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. Web using a simple, general trend for the ionic charge for elements on the periodic table, in this video we find the ionic charge for. But the question is how can you find the ionic charge on aluminum (al)?. Predict which.
What is the Charge on the Ion Formed by Aluminum? Aluminum Profile Blog
Web aluminum and carbon react to form an ionic compound. For example, iron( ii ) has a. Web the charge of an aluminum ion is typically 3+. Web the ionic charge of aluminum (al) is 3+. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge.
Predict which forms an anion, which forms a cation, and the. Web the ionic charge of aluminum (al) is 3+. Web roman numeral notation indicates charge of ion when element commonly forms more than one ion. For example, iron( ii ) has a. Three fluorine 1− ions are needed. Web aluminum and carbon react to form an ionic compound. Web the charge of an aluminum ion is typically 3+. Web using a simple, general trend for the ionic charge for elements on the periodic table, in this video we find the ionic charge for. This is because the element's atomic number is 13, reflecting the fact that it has. But the question is how can you find the ionic charge on aluminum (al)?. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Web the aluminum ion has a 3+ charge, while the fluoride ion formed by fluorine has a 1− charge. Web aluminum and carbon react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each.
Web The Charge Of An Aluminum Ion Is Typically 3+.
Web using a simple, general trend for the ionic charge for elements on the periodic table, in this video we find the ionic charge for. But the question is how can you find the ionic charge on aluminum (al)?. Web the ionic charge of aluminum (al) is 3+. This is because the element's atomic number is 13, reflecting the fact that it has.
Web Roman Numeral Notation Indicates Charge Of Ion When Element Commonly Forms More Than One Ion.
Predict which forms an anion, which forms a cation, and the charges of each. Predict which forms an anion, which forms a cation, and the. Web aluminum and carbon react to form an ionic compound. Web aluminum and carbon react to form an ionic compound.
Web The Aluminum Ion Has A 3+ Charge, While The Fluoride Ion Formed By Fluorine Has A 1− Charge.
For example, iron( ii ) has a. Three fluorine 1− ions are needed. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge.