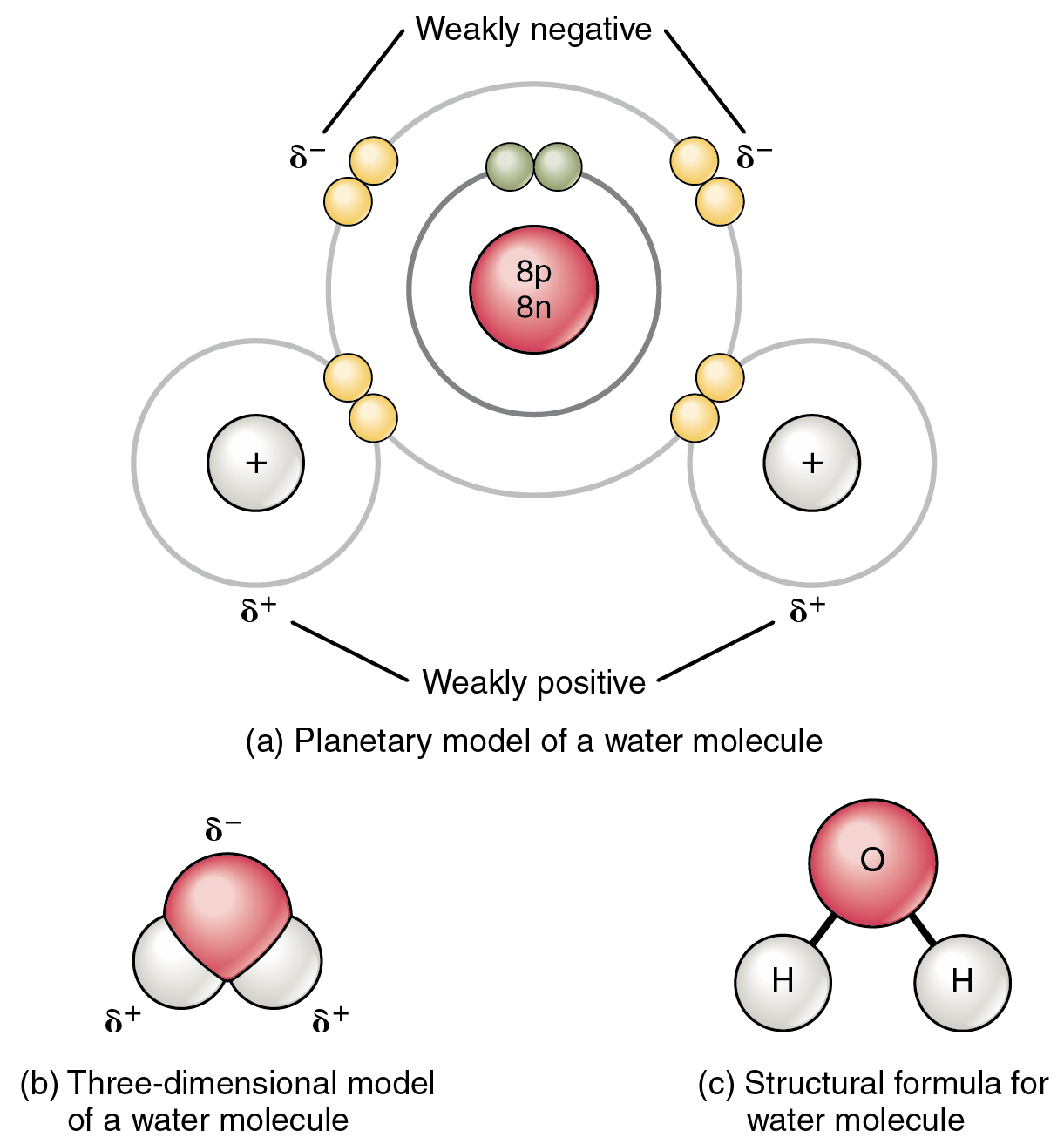
Which Combination Of Atoms Can Form A Polar Covalent Bond
Which Combination Of Atoms Can Form A Polar Covalent Bond - Web as a rough guide, bonds between atoms whose electronegativities differ by less than 0.5 are nonpolar covalent, bonds between. Web a bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a. The polarity of such a. There is a bond between the atoms, and electrons are shared, but they are pulled more closely towards one. Web compounds with polar covalent bonds have electrons that are shared unequally between the bonded atoms. Web compounds with polar covalent bonds have electrons that are shared unequally between the bonded. Web a polar covalent bond is created when the oxygen (o) atom, more electronegative than hydrogen, pulls the shared electrons towards itself. Web in a polar bond, two atoms share electrons unevenly. Web which combination of atoms can form a polar covalent bond?
Attractive Forces and Bonds
Web a bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a. Web compounds with polar covalent bonds have electrons that are shared unequally between the bonded atoms. Web compounds with polar covalent bonds have electrons that are shared unequally between the bonded. The polarity of such a. Web which combination of atoms.
Polar Covalent Bond Definitions, Types and Examples
Web compounds with polar covalent bonds have electrons that are shared unequally between the bonded. Web in a polar bond, two atoms share electrons unevenly. Web compounds with polar covalent bonds have electrons that are shared unequally between the bonded atoms. Web a polar covalent bond is created when the oxygen (o) atom, more electronegative than hydrogen, pulls the shared.
Which Pair Of Atoms Forms The Most Polar Bond
Web a polar covalent bond is created when the oxygen (o) atom, more electronegative than hydrogen, pulls the shared electrons towards itself. Web which combination of atoms can form a polar covalent bond? Web a bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a. Web compounds with polar covalent bonds have electrons.
Polar Bond Covalent Bonding Molecules Organic Chemist vrogue.co
Web which combination of atoms can form a polar covalent bond? There is a bond between the atoms, and electrons are shared, but they are pulled more closely towards one. Web compounds with polar covalent bonds have electrons that are shared unequally between the bonded. Web a polar covalent bond is created when the oxygen (o) atom, more electronegative than.
Polar Covalent Bonds
Web compounds with polar covalent bonds have electrons that are shared unequally between the bonded. Web which combination of atoms can form a polar covalent bond? There is a bond between the atoms, and electrons are shared, but they are pulled more closely towards one. Web in a polar bond, two atoms share electrons unevenly. Web a polar covalent bond.
Polar Covalent Bond Definition and Examples in Chemistry
Web a polar covalent bond is created when the oxygen (o) atom, more electronegative than hydrogen, pulls the shared electrons towards itself. Web in a polar bond, two atoms share electrons unevenly. The polarity of such a. Web compounds with polar covalent bonds have electrons that are shared unequally between the bonded. Web which combination of atoms can form a.
PPT Bond Polarity and Molecules PowerPoint Presentation, free download ID1717832
Web compounds with polar covalent bonds have electrons that are shared unequally between the bonded atoms. Web in a polar bond, two atoms share electrons unevenly. Web a polar covalent bond is created when the oxygen (o) atom, more electronegative than hydrogen, pulls the shared electrons towards itself. Web as a rough guide, bonds between atoms whose electronegativities differ by.
Polar vs. Nonpolar Bonds — Overview & Examples Expii Covalent bonding, Bond, Chemistry quotes
There is a bond between the atoms, and electrons are shared, but they are pulled more closely towards one. The polarity of such a. Web as a rough guide, bonds between atoms whose electronegativities differ by less than 0.5 are nonpolar covalent, bonds between. Web which combination of atoms can form a polar covalent bond? Web in a polar bond,.
Polar Covalent Bonds Clearly Explained for Easy Learning
Web compounds with polar covalent bonds have electrons that are shared unequally between the bonded atoms. Web a polar covalent bond is created when the oxygen (o) atom, more electronegative than hydrogen, pulls the shared electrons towards itself. Web which combination of atoms can form a polar covalent bond? The polarity of such a. Web in a polar bond, two.
Polar Covalent Bonds Clearly Explained for Easy Learning
Web a polar covalent bond is created when the oxygen (o) atom, more electronegative than hydrogen, pulls the shared electrons towards itself. Web as a rough guide, bonds between atoms whose electronegativities differ by less than 0.5 are nonpolar covalent, bonds between. There is a bond between the atoms, and electrons are shared, but they are pulled more closely towards.
Web as a rough guide, bonds between atoms whose electronegativities differ by less than 0.5 are nonpolar covalent, bonds between. Web in a polar bond, two atoms share electrons unevenly. The polarity of such a. Web a polar covalent bond is created when the oxygen (o) atom, more electronegative than hydrogen, pulls the shared electrons towards itself. Web compounds with polar covalent bonds have electrons that are shared unequally between the bonded atoms. Web a bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a. Web compounds with polar covalent bonds have electrons that are shared unequally between the bonded. Web which combination of atoms can form a polar covalent bond? There is a bond between the atoms, and electrons are shared, but they are pulled more closely towards one.
There Is A Bond Between The Atoms, And Electrons Are Shared, But They Are Pulled More Closely Towards One.
Web compounds with polar covalent bonds have electrons that are shared unequally between the bonded. Web which combination of atoms can form a polar covalent bond? Web as a rough guide, bonds between atoms whose electronegativities differ by less than 0.5 are nonpolar covalent, bonds between. The polarity of such a.
Web In A Polar Bond, Two Atoms Share Electrons Unevenly.
Web a polar covalent bond is created when the oxygen (o) atom, more electronegative than hydrogen, pulls the shared electrons towards itself. Web compounds with polar covalent bonds have electrons that are shared unequally between the bonded atoms. Web a bond in which the electronegativity difference between the atoms is between 0.5 and 2.1 is called a.