Which Group Tends Not To Form Ions Or React
Which Group Tends Not To Form Ions Or React - Nuclear charge, atomic radius, shielding effect and sublevel arrangement. Magnesium and nitrogen react to form an ionic compound. Be able to defend your. Web conversely, elements in groups 17, 16, and 15 often react to gain one, two, and three electrons, respectively, to form ions such. Which group tends to form 1+ ions? Web four major factors affect reactivity of metals: Web based on the concept of periodic trends, answer the following questions for these atoms: Click the card to flip 👆. Click the card to flip 👆.
Solved Ions and the Periodic Table A main group metal tends
Click the card to flip 👆. Web based on the concept of periodic trends, answer the following questions for these atoms: Web four major factors affect reactivity of metals: Click the card to flip 👆. Web conversely, elements in groups 17, 16, and 15 often react to gain one, two, and three electrons, respectively, to form ions such.
Molecular and Ionic Compounds Chemistry I
Web four major factors affect reactivity of metals: Click the card to flip 👆. Which group tends to form 1+ ions? Web based on the concept of periodic trends, answer the following questions for these atoms: Magnesium and nitrogen react to form an ionic compound.
PPT Chapter 6 The Periodic Table PowerPoint Presentation, free download ID5955375
Click the card to flip 👆. Be able to defend your. Web four major factors affect reactivity of metals: Which group tends to form 1+ ions? Web based on the concept of periodic trends, answer the following questions for these atoms:
PPT Atoms, Molecules and Ions PowerPoint Presentation, free download ID2087812
Which group tends to form 1+ ions? Nuclear charge, atomic radius, shielding effect and sublevel arrangement. Web conversely, elements in groups 17, 16, and 15 often react to gain one, two, and three electrons, respectively, to form ions such. Be able to defend your. Click the card to flip 👆.
CH150 Chapter 3 Ions and Ionic Compounds Chemistry
Which group tends to form 1+ ions? Be able to defend your. Web four major factors affect reactivity of metals: Nuclear charge, atomic radius, shielding effect and sublevel arrangement. Magnesium and nitrogen react to form an ionic compound.
Ionic Compound Nomenclature Presentation Chemistry
Be able to defend your. Web four major factors affect reactivity of metals: Nuclear charge, atomic radius, shielding effect and sublevel arrangement. Which group tends to form 1+ ions? Magnesium and nitrogen react to form an ionic compound.
PPT The Representative Elements PowerPoint Presentation, free download ID2315939
Click the card to flip 👆. Magnesium and nitrogen react to form an ionic compound. Click the card to flip 👆. Web based on the concept of periodic trends, answer the following questions for these atoms: Which group tends to form 1+ ions?
CH150 Chapter 3 Ions and Ionic Compounds Chemistry
Magnesium and nitrogen react to form an ionic compound. Which group tends to form 1+ ions? Click the card to flip 👆. Nuclear charge, atomic radius, shielding effect and sublevel arrangement. Web based on the concept of periodic trends, answer the following questions for these atoms:
Chapter 10.3 Naming Ionic Compounds Chemistry LibreTexts
Web based on the concept of periodic trends, answer the following questions for these atoms: Web conversely, elements in groups 17, 16, and 15 often react to gain one, two, and three electrons, respectively, to form ions such. Be able to defend your. Nuclear charge, atomic radius, shielding effect and sublevel arrangement. Magnesium and nitrogen react to form an ionic.
CH104 Chapter 3 Ions and Ionic Compounds Chemistry
Click the card to flip 👆. Web conversely, elements in groups 17, 16, and 15 often react to gain one, two, and three electrons, respectively, to form ions such. Magnesium and nitrogen react to form an ionic compound. Be able to defend your. Which group tends to form 1+ ions?
Which group tends to form 1+ ions? Click the card to flip 👆. Nuclear charge, atomic radius, shielding effect and sublevel arrangement. Web conversely, elements in groups 17, 16, and 15 often react to gain one, two, and three electrons, respectively, to form ions such. Web based on the concept of periodic trends, answer the following questions for these atoms: Be able to defend your. Web four major factors affect reactivity of metals: Magnesium and nitrogen react to form an ionic compound. Click the card to flip 👆.
Web Based On The Concept Of Periodic Trends, Answer The Following Questions For These Atoms:
Which group tends to form 1+ ions? Web conversely, elements in groups 17, 16, and 15 often react to gain one, two, and three electrons, respectively, to form ions such. Magnesium and nitrogen react to form an ionic compound. Click the card to flip 👆.
Web Four Major Factors Affect Reactivity Of Metals:
Click the card to flip 👆. Be able to defend your. Nuclear charge, atomic radius, shielding effect and sublevel arrangement.





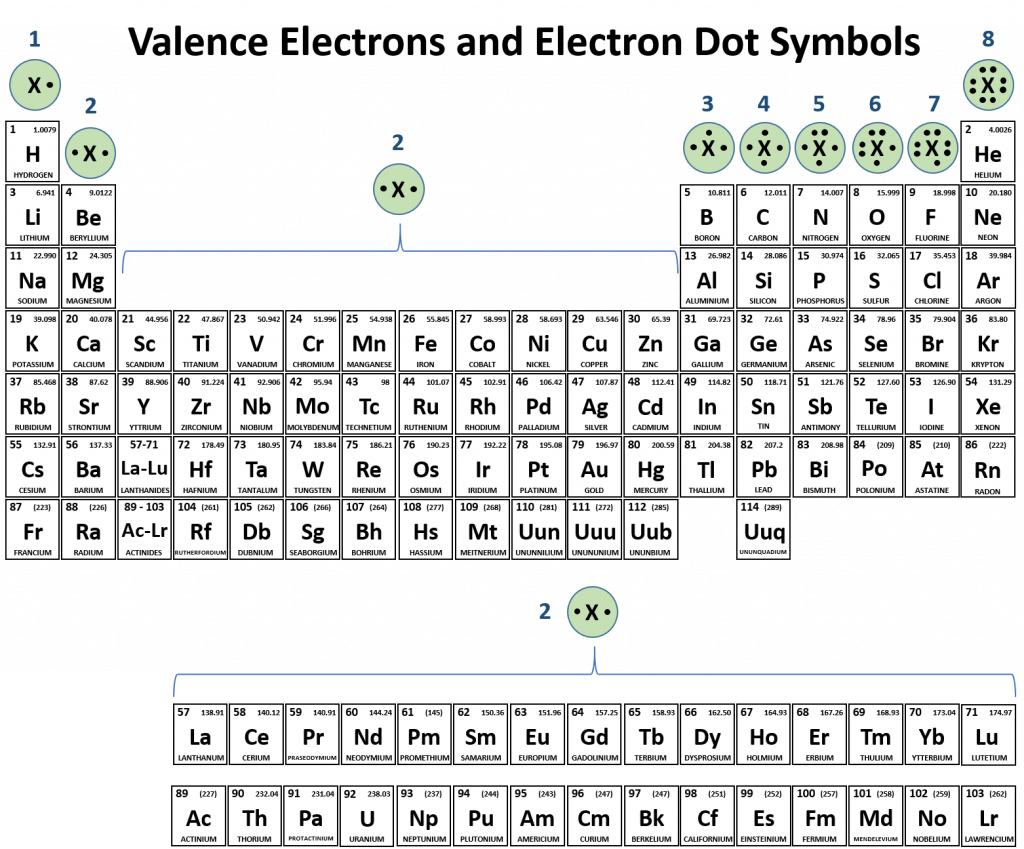
.PNG)



