Which Group Tends To Form 2+ Ions
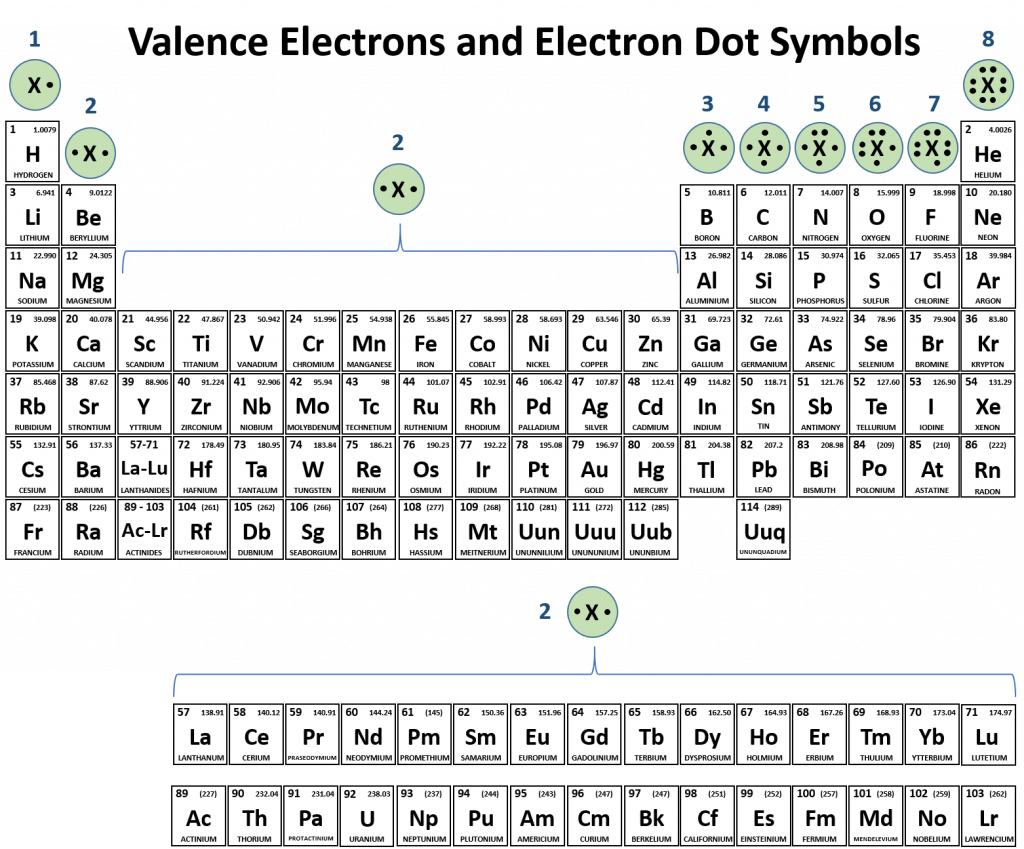
Which Group Tends To Form 2+ Ions - Web group 1 metals, the alkali metals, have the 1 valence electron, and thus form m + ions when oxidized. Group 16 elements (two groups left) form 2−. Web there also exists a group of ions that contain more than one atom. Web for example, group 17 elements (one group left of the noble gases) form 1− ions; These are called polyatomic ions.
AQA A Level Chemistry复习笔记4.2.2 Identifying Anions & Cations翰林国际教育
Web group 1 metals, the alkali metals, have the 1 valence electron, and thus form m + ions when oxidized. These are called polyatomic ions. Web there also exists a group of ions that contain more than one atom. Web for example, group 17 elements (one group left of the noble gases) form 1− ions; Group 16 elements (two groups.
Ionic Compound Nomenclature Presentation Chemistry
These are called polyatomic ions. Web for example, group 17 elements (one group left of the noble gases) form 1− ions; Group 16 elements (two groups left) form 2−. Web there also exists a group of ions that contain more than one atom. Web group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +.
CH150 Chapter 3 Ions and Ionic Compounds Chemistry
These are called polyatomic ions. Web there also exists a group of ions that contain more than one atom. Group 16 elements (two groups left) form 2−. Web group 1 metals, the alkali metals, have the 1 valence electron, and thus form m + ions when oxidized. Web for example, group 17 elements (one group left of the noble gases).
Solved Divide the ions below into 2 group, those that tend
Group 16 elements (two groups left) form 2−. Web group 1 metals, the alkali metals, have the 1 valence electron, and thus form m + ions when oxidized. Web there also exists a group of ions that contain more than one atom. Web for example, group 17 elements (one group left of the noble gases) form 1− ions; These are.
PPT Atoms, Molecules and Ions PowerPoint Presentation, free download ID2087812
Web for example, group 17 elements (one group left of the noble gases) form 1− ions; Group 16 elements (two groups left) form 2−. Web group 1 metals, the alkali metals, have the 1 valence electron, and thus form m + ions when oxidized. Web there also exists a group of ions that contain more than one atom. These are.
CH104 Chapter 3 Ions and Ionic Compounds Chemistry
Web for example, group 17 elements (one group left of the noble gases) form 1− ions; These are called polyatomic ions. Group 16 elements (two groups left) form 2−. Web group 1 metals, the alkali metals, have the 1 valence electron, and thus form m + ions when oxidized. Web there also exists a group of ions that contain more.
SOLVEDUse the electron configuration of oxygen to explain why it tends to form a 2 ion.
These are called polyatomic ions. Web group 1 metals, the alkali metals, have the 1 valence electron, and thus form m + ions when oxidized. Web there also exists a group of ions that contain more than one atom. Web for example, group 17 elements (one group left of the noble gases) form 1− ions; Group 16 elements (two groups.
PPT Chapter 9 “Chemical Names and Formulas” PowerPoint Presentation ID545924
These are called polyatomic ions. Group 16 elements (two groups left) form 2−. Web there also exists a group of ions that contain more than one atom. Web group 1 metals, the alkali metals, have the 1 valence electron, and thus form m + ions when oxidized. Web for example, group 17 elements (one group left of the noble gases).
PPT Ions and Ionic Bonding PowerPoint Presentation, free download ID2962626
Group 16 elements (two groups left) form 2−. Web for example, group 17 elements (one group left of the noble gases) form 1− ions; These are called polyatomic ions. Web group 1 metals, the alkali metals, have the 1 valence electron, and thus form m + ions when oxidized. Web there also exists a group of ions that contain more.
Periodic Table PT. ppt download
Web there also exists a group of ions that contain more than one atom. These are called polyatomic ions. Group 16 elements (two groups left) form 2−. Web for example, group 17 elements (one group left of the noble gases) form 1− ions; Web group 1 metals, the alkali metals, have the 1 valence electron, and thus form m +.
Web there also exists a group of ions that contain more than one atom. Web group 1 metals, the alkali metals, have the 1 valence electron, and thus form m + ions when oxidized. Group 16 elements (two groups left) form 2−. Web for example, group 17 elements (one group left of the noble gases) form 1− ions; These are called polyatomic ions.
These Are Called Polyatomic Ions.
Group 16 elements (two groups left) form 2−. Web group 1 metals, the alkali metals, have the 1 valence electron, and thus form m + ions when oxidized. Web for example, group 17 elements (one group left of the noble gases) form 1− ions; Web there also exists a group of ions that contain more than one atom.


.PNG)